silicon atom|Silicon Facts (Atomic Number 14 or Si) : Bacolod CompoundsMost silicon is used industrially without being purified, often with comparatively little . Tingnan ang higit pa Discover the thrilling Aviator game on Betway South Africa, a unique and popular social multiplayer casino game brought to you by Spribe. Get ready to soar to new heights as you enjoy this easy-to-understand crash-style casino game that is perfect for both beginners and advanced players.
PH0 · silicon summary
PH1 · WebElements Periodic Table » Silicon » the essentials
PH2 · Silicon Facts (Atomic Number 14 or Si)
PH3 · Silicon (Si) [14] — Chemical Element — Periodic Table
PH4 · Silicon (Si)
PH5 · Silicon
Cкачать лучшие игры торрент репаки от Механики! Знакомьтесь с repack-games.ru — именно здесь можно скачать с торрента самые лучшие игры от легендарной команды Механиков!Is BGO Casino real or fake. Read review BGO Casino casino. Casino reviews and bonus codes. We've thoroughly reviewed BGO Casino and gave it a very good reputation rating, which means it's a great casino to play at. In our review, we've considered the casino's player complaints, estimated revenues, license, games genuineness, customer support .
silicon atom*******Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below . Tingnan ang higit paOwing to the abundance of silicon in the Earth's crust, natural silicon-based materials have been used for thousands of years. Silicon rock crystals were familiar to various Tingnan ang higit paCrystalline bulk silicon is rather inert, but becomes more reactive at high temperatures. Like its neighbour aluminium, silicon forms a thin, continuous surface layer of silicon dioxide (SiO 2) that protects the metal from oxidation. Thus silicon . Tingnan ang higit paCompoundsMost silicon is used industrially without being purified, often with comparatively little . Tingnan ang higit paAlthough silicon is readily available in the form of silicates, very few organisms use it directly. Diatoms, radiolaria, and siliceous sponges Tingnan ang higit paPhysical and atomicA silicon atom has fourteen electrons. In the ground state, they are arranged in the electron . Tingnan ang higit paSilicon is the eighth most abundant element in the universe, coming after hydrogen, helium, carbon, nitrogen, oxygen, iron, and neon. These abundances . Tingnan ang higit pa
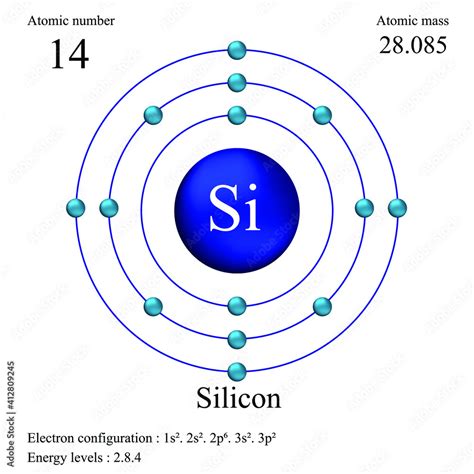
Silicon of 96–99% purity is made by carbothermically reducing quartzite or sand with highly pure coke. The reduction is carried out in an electric arc furnace, with an . Tingnan ang higit paSilicon is a nonmetallic chemical element in the carbon family, the second most abundant in Earth's crust. It forms compounds with oxygen as silica and silicates, .Learn about silicon, the second most abundant element in the Earth's crust, and its applications in alloys, silicones, semiconductors, glass and more. Find out its history, discovery, biological .Silicon is the 14th element in the periodic table and has a symbol of Si and atomic number of 14. It has an atomic weight of 28.085 and a mass number of 28. Silicon has fourteen protons and .Learn about silicon, the second most abundant element in earth's crust and a key component of semiconductors. Find out how silicon was discovered, what are its properties, uses, health effects and isotopes.Hul 3, 2019 — Learn about the chemical and physical properties, uses, sources, and history of silicon, a metalloid element with atomic number 14 and symbol Si. Find out how silicon is important for life, electronics, and the universe.Silicon is a nonmetallic to semimetallic element with atomic number 14 and symbol Si. It is abundant in Earth's crust and has many applications in electronics, metallurgy, and glassmaking.In 1800, Sir Humphry Davy thought silica to be a compound and not an element; but in 1811, Gay Lussac and Louis Jacques Thénard probably prepared impure amorphous silicon by heating potassium with silicon tetrafluoride.Silicon is a metalloid with atomic number 14 and symbol Si. It is the second most abundant element in the earth's crust and has various applications in glass, ceramics, and semiconductors.Silicon atoms have 14 electrons and the shell structure is 2.8.4. The ground state electronic configuration of neutral silicon is [ Ne ]. 3s2. 3p2 and the term symbol of silicon is 3P0. Silicon: .
Nob 21, 2020 — The atomic radius of Silicon atom is 111pm (covalent radius). It must be noted, atoms lack a well-defined outer boundary. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in .Silicon atoms have 14 electrons and the shell structure is 2.8.4. The ground state electronic configuration of neutral silicon is [Ne].3s 2.3p 2 and the term symbol of silicon is 3 P 0. Silicon: description Your user agent does not support the HTML5 Audio element.Ago 14, 2020 — An atom of the alkaline earth metal beryllium, with an atomic number of 4, contains four protons in the nucleus and four electrons surrounding the nucleus. . The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon .Mar 17, 2023 — This electron configuration shows that the last shell of the silicon atom has two unpaired electrons. So the valency of silicon is 2. When silicon atoms are excited, silicon atoms absorb energy. As a result, an electron in the 3s orbital jumps to the 3p z orbital. The second orbit of the silicon atom is filled with electrons.
That's because the hydroxy group on the first silicon atom is still a nucleophile, and it could donate to a second silicon atom, forming a bridge between two silicon atoms. If the water is introduced gradually to the dichlorosilane, polymerization results, because a chlorosilane is more likely to react with a neighbouring silanol than with .Abr 27, 2018 — Atomic number (number of protons in the nucleus): 14; Atomic symbol (on the Periodic Table of Elements): Si; Atomic weight (average mass of the atom): 28.09; Density: 2.3296 grams per cubic centimeter
In 1800, Sir Humphry Davy thought silica to be a compound and not an element; but in 1811, Gay Lussac and Louis Jacques Thénard probably prepared impure amorphous silicon by heating potassium with silicon tetrafluoride. In 1824 Jöns Jakob Berzelius prepared amorphous silicon by the same general method.
silicon atomIn 1800, Sir Humphry Davy thought silica to be a compound and not an element; but in 1811, Gay Lussac and Louis Jacques Thénard probably prepared impure amorphous silicon by heating potassium with silicon tetrafluoride. In 1824 Jöns Jakob Berzelius prepared amorphous silicon by the same general method.Nob 21, 2020 — Silicon is a chemical element with atomic number 14 which means there are 14 protons and 14 electrons in the atomic structure. The chemical symbol for Silicon is Si . Silicon is a hard and brittle crystalline solid with a blue-grey metallic lustre, it is a .Silicon atoms have 14 electrons and the shell structure is 2.8.4. The ground state electron configuration of ground state gaseous neutral silicon is [Ne].3s 2.3p 2 and the term symbol is 3 P 0. Schematic electronic configuration of silicon. The Kossel shell structure of silicon.

What is the structure of silicon(IV) oxide? Extended tier only. Silicon(IV) oxide (also known as silicon dioxide or silica), SiO 2, is a macromolecular compound which occurs naturally as sand and quartz; Each oxygen atom forms covalent bonds with 2 silicon atoms and each silicon atom in turn forms covalent bonds with 4 oxygen atoms; A tetrahedron is formed with one .Abr 22, 2024 — Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle, gray-white metalloid that belongs to the group 14 elements in the periodic table. About 90% of the minerals that make up the .Silicon Facts (Atomic Number 14 or Si) Get the facts about element Silicon (Si) [14] from the periodic table. Find physical data, electron configuration, chemical properties, aggregation states, isotope data (including decay trees) as well as some historic information. . Periodic Table of the Elements; Silicon: Metalloid: Symbol: Si Atomic number: 14 Atomic mass: 28.0855 Group .silicon atom Silicon Facts (Atomic Number 14 or Si) Get the facts about element Silicon (Si) [14] from the periodic table. Find physical data, electron configuration, chemical properties, aggregation states, isotope data (including decay trees) as well as some historic information. . Periodic Table of the Elements; Silicon: Metalloid: Symbol: Si Atomic number: 14 Atomic mass: 28.0855 Group .
Nob 21, 2020 — The atomic radius of Silicon atom is 111pm (covalent radius). It must be noted, atoms lack a well-defined outer boundary. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in .Silicon is most commonly found in silicate compounds. Silica is the one stable oxide of silicon, and has the empirical formula SiO 2. Silica is not a silicon atom with two double bonds to two oxygen atoms. Silica is composed of one silicon atom with four single bonds to four oxygen molecules (Figure 2). Figure 2: The net charge of silica is minus 4
silicon. Formula: Si; Molecular weight: 28.0855; IUPAC Standard InChI: InChI=1S/Si Copy. IUPAC Standard InChIKey: XUIMIQQOPSSXEZ-UHFFFAOYSA-N Copy; CAS Registry Number: 7440-21-3; . NIST Atomic Spectra Database - Lines Holdings (on physics web site) NIST Atomic Spectra Database - Levels Holdings (on physics web site) .Silicium, oder auch Silizium, [13] ist ein chemisches Element mit dem Symbol Si und der Ordnungszahl 14. Es steht in der 4. Hauptgruppe (Kohlenstoffgruppe), bzw. der 14. IUPAC-Gruppe, und der 3.Periode des Periodensystems der Elemente.In der Erdhülle ist es, auf den Massenanteil bezogen, nach Sauerstoff das zweithäufigste Element.. Silicium ist ein .
silicon, Nonmetallic to semimetallic chemical element, chemical symbol Si, atomic number 14.Second only to oxygen in abundance in Earth’s crust, it never occurs free but is found in almost all rocks and in sand, clay, and soils, combined with oxygen as silica (silicon dioxide, SiO 2) or with oxygen and metals as silicate minerals.It occurs in many plants and some animals.A good example is doping silicon with phosphorus. Here, there’s an excess of electron charge carriers. This is because the doping phosphorus atom has one more valence electron than the host silicon atoms. N-Type Semiconductor Source: Lumen learning. A silicon atom has 4 electrons bound tightly to its outer orbit. Phosphorus has 5 electrons .
GFAFX 0.67%: Lipper Large-Cap Growth Funds Average 0.99% : Fund as of most recent prospectus. Lipper Category as of 6/30/24 (updated quarterly). Resources. Literature Quarterly Fund Fact Sheet (PDF) 07/24. Quarterly Attribution Report (PDF) 07/24 .
silicon atom|Silicon Facts (Atomic Number 14 or Si)